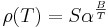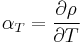Temperature coefficient
The temperature coefficient is the relative change of a physical property when the temperature is changed by 1 K.
In the following formula, let R be the physical property to be measured and T be the temperature at which the property is measured. T0 is the reference temperature, and ΔT is the difference between T and T0. Finally, α is the (linear) temperature coefficient. Given these definitions, the physical property is:
Here α has the dimensions of an inverse temperature (1/K or K−1).
This equation is linear with respect to temperature. For quantities that vary polynomially or logarithmically with temperature, it may be possible to calculate a temperature coefficient that is a useful approximation for a certain range of temperatures. For quantities that vary exponentially with temperature, such as the rate of a chemical reaction, any temperature coefficient would be valid only over a very small temperature range.
Different temperature coefficients are specified for various applications, including nuclear, electrical and magnetic.
Contents |
Negative temperature coefficient
A negative temperature coefficient (NTC) occurs when the thermal conductivity of a material rises with increasing temperature, typically in a defined temperature range. For most materials, the thermal conductivity will decrease with increasing temperature.
Materials with a negative temperature coefficient have been used in floor heating since 1971. The negative temperature coefficient avoids excessive local heating beneath carpets, bean bag chairs, mattresses etc., which can damage wooden floors, and may infrequently cause fires.
Most ceramics exhibit NTC behaviour, which is governed by an Arrhenius equation over a wide range of temperatures:
where R is resistance, A and B are constants, and T is absolute temperature (K). The constant B is related to the energies required to form and move the charge carriers responsible for electrical conduction – hence, as the value of B decreases, the material becomes insulating. Practical and commercial NTC resistors aim to combine modest resistance with a value of B that provides good sensitivity to temperature. Such is the importance of the B constant value, that it is possible to characterize NTC thermistors using the B parameter equation:
where 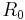 is resistance at temperature
is resistance at temperature 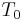 . Therefore, many materials that produce acceptable values of
. Therefore, many materials that produce acceptable values of 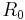 include materials that have been alloyed or possess variable cation valence states and thus contain a high natural defect center concentration. The value of B strongly depends on the energy required to dissociate the charge carriers that are used for the electrical conduction from these defect centers.
include materials that have been alloyed or possess variable cation valence states and thus contain a high natural defect center concentration. The value of B strongly depends on the energy required to dissociate the charge carriers that are used for the electrical conduction from these defect centers.
Reversible temperature coefficient
Residual magnetic flux density or Br changes with temperature and it is one of the important characteristics of magnet performance. Some applications, such as interial gyroscopes and traveling-wave tubes (TWTs), need to have constant field over a wide temperature range. The reversible temperature coefficient (RTC) of Br is defined as:
To address these requirements, temperature compensated magnets were developed in the late 1970s.[1] For conventional SmCo magnets, Br decreases as temperature increases. Conversely, for GdCo magnets, Br increases as temperature increases within certain temperature ranges. By combining samarium and gadolinium in the alloy, the temperature coefficient can be reduced to nearly zero.
Temperature coefficient of electrical resistance
The temperature dependence of electrical resistance and thus of electronic devices (wires, resistors) has to be taken into account when constructing devices and circuits. The temperature dependence of conductors is to a great degree linear and can be described by the approximation below.
where
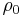 just corresponds to the specific resistance temperature coefficient at a specified reference value (normally T = 0 °C)[2]
just corresponds to the specific resistance temperature coefficient at a specified reference value (normally T = 0 °C)[2]
That of a semiconductor is however exponential:
where 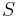 is defined as the cross sectional area and
is defined as the cross sectional area and  and
and 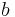 are coefficients determining the shape of the function and the value of resistivity at a given temperature.
are coefficients determining the shape of the function and the value of resistivity at a given temperature.
For both,  is referred to as the resistance temperature coefficient.[3]
is referred to as the resistance temperature coefficient.[3]
This property is used in devices such as thermistors.
Positive temperature coefficient of resistance
A positive temperature coefficient (PTC) refers to materials that experience an increase in electrical resistance when their temperature is raised. Materials which have useful engineering applications usually show a relatively rapid increase with temperature, i.e. a higher coefficient. The higher the coefficient, the greater an increase in electrical resistance for a given temperature increase.
Coefficient of thermal expansion
The physical dimensions of matter can be affected by temperature. The coefficient of thermal expansion for a given sample of matter can be used to approximate its change in volume given a change in temperature. A similar coefficient, the linear thermal expansion coefficient, is also often used to measure the change of length of an object in one-dimension.
The coefficient of thermal expansion is often used to develop thermometers. Here lengths of materials can express temperature. The coefficient is also used for several types of thermostats.
Temperature coefficient of elasticity
The elastic modulus of elastic materials varies with temperature, typically decreasing with higher temperature.
Temperature coefficient of reactivity
In nuclear engineering, the temperature coefficient of reactivity is a measure of the change in reactivity (resulting in a change in power), brought about by a change in temperature of the reactor components or the reactor coolant. This may be defined as
Where 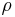 is reactivity and T is temperature. The relationship shows that
is reactivity and T is temperature. The relationship shows that 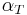 is the value of the partial differential of reactivity with respect to temperature and is referred to as the "temperature coefficient of reactivity". As a result, the temperature feedback provided by
is the value of the partial differential of reactivity with respect to temperature and is referred to as the "temperature coefficient of reactivity". As a result, the temperature feedback provided by 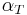 has an intuitive application to passive nuclear safety. A negative
has an intuitive application to passive nuclear safety. A negative 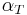 is broadly cited as important for reactor safety, but wide temperature variations across real reactors (as opposed to a theoretical homogeneous reactor) limit the usability of a single metric as a marker of reactor safety.[4]
is broadly cited as important for reactor safety, but wide temperature variations across real reactors (as opposed to a theoretical homogeneous reactor) limit the usability of a single metric as a marker of reactor safety.[4]
In water moderated nuclear reactors, the bulk of reactivity changes with respect to temperature are brought about by changes in the temperature of the water. However each element of the core has a specific temperature coefficient of reactivity (e.g. the fuel or cladding). The mechanisms which drive fuel temperature coefficients of reactivity are different than water temperature coefficients. While water expands as temperature increases, causing longer neutron travel times during moderation, fuel material will not expand appreciably. Changes in reactivity in fuel due to temperature stem from a phenomenon known as doppler broadening, where resonance absorption of fast neutrons in fuel filler material prevents those neutrons from thermalizing (slowing down).[5]
Units
The thermal coefficient of electrical circuit parts is sometimes specified as ppm/°C. This specifies the percentage (expressed in parts per million) that its electrical characteristics will deviate when taken to a temperature above or below the operating temperature.
References
- ^ "About Us". Electron Energy Corporation. http://www.electronenergy.com/about-us/about-us.htm.
- ^ Kasap, S. O. (2006). Principles of Electronic Materials and Devices (Third ed.). Mc-Graw Hill. p. 126.
- ^ Alenitsyn, Alexander G.; Butikov, Eugene I.; Kondraryez, Alexander S. (1997). Concise Handbook of Mathematics and Physics. CRC Press. pp. 331–332. ISBN 0849377455.
- ^ Duderstadt & Hamilton 1976, pp. 259–261
- ^ Duderstadt & Hamilton 1976, pp. 556–559
Bibliography
- Duderstadt, James J.; Hamilton, Louis J. (1976). Nuclear Reactor Analysis. Wiley. ISBN 0471223638.
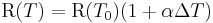
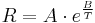
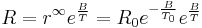
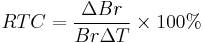
![\operatorname{\rho}(T) = \rho_{0}[1 %2B \alpha_{0}(T-T_{0})]](/2012-wikipedia_en_all_nopic_01_2012/I/34dcd390ed601b65d38f940ded1376da.png)
![\alpha_{0}=\frac{1}{\rho_{0}}\left [ \frac{\delta \rho}{\delta T}\right ]_{T=T_{0}}](/2012-wikipedia_en_all_nopic_01_2012/I/a3af74114d352eb054259a7e7836b10e.png)